
As the medical device industry evolves, so does the regulatory landscape. Companies face increasing scrutiny from regulatory bodies like the FDA, which demands robust compliance and data integrity programs to ensure patient safety and product efficacy. Two critical terms often discussed in this realm are CSV (Computer System Validation) and CSA (Computer Software Assurance). This blog post by Compliance Gurus dives deep into the distinctions between CSV and CSA, explores data integrity programs and assessments, and highlights key concepts like FDA remediation and ALCOA+ principles in software validation. Understanding these aspects is crucial for any medical device manufacturer or software developer navigating FDA regulations in 2025 and beyond.
Understanding CSV and CSA: Definitions and Applications
What is CSV (Computer System Validation)?
Computer System Validation (CSV) is a traditional, well-established approach used in regulated industries, including medical devices, to ensure that software systems meet predefined requirements and function correctly. CSV involves a comprehensive, documented process of planning, testing, and verifying software systems to confirm compliance with regulatory standards such as FDA 21 CFR Part 11.
Key elements of CSV include:
Detailed requirement specifications
Verification and validation testing
Documentation of results
Traceability matrices
Change control processes
What is CSA (Computer Software Assurance)?
Computer Software Assurance (CSA) is a modernized approach promoted by the FDA to streamline the validation process, focusing on risk management and leveraging automated testing and modern development practices. CSA shifts emphasis from exhaustive documentation toward evidence-based assurance, prioritizing software quality and patient safety through intelligent risk assessment.
CSA benefits include:
Risk-based focus
Less prescriptive documentation requirements
Increased reliance on automation and continuous monitoring
Faster time to market
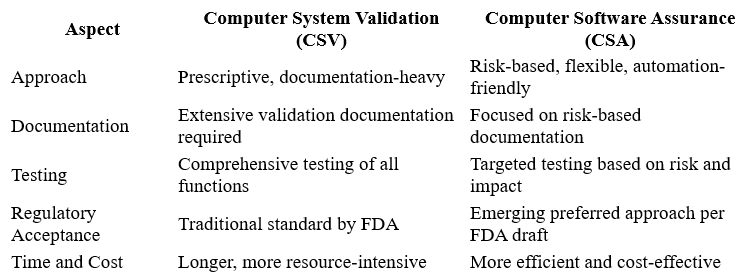
CSV vs CSA for Medical Devices: What’s the Difference?
1. Approach to Validation
CSV: Prescriptive and documentation-heavy; requires extensive testing of all software functions regardless of risk.
CSA: Risk-based and flexible; testing and documentation efforts are aligned with the risk associated with the software’s use and impact on patient safety.
2. Regulatory Focus
CSV: FDA guidance has traditionally emphasized CSV, making it the standard approach.
CSA: The FDA’s new draft guidance encourages CSA, reflecting the agency’s acceptance of newer technologies and validation philosophies.
3. Documentation and Evidence
CSV: Requires comprehensive and formal documentation including validation plans, test scripts, and reports.
CSA: Documentation is focused on critical controls and risk assessments rather than exhaustive test execution.
4. Time and Resource Efficiency
CSV: Often resource-intensive and time-consuming, which can delay product release.
CSA: Designed to be more efficient, reducing unnecessary work while maintaining compliance.
Why Data Integrity Programs and Assessments Are Critical
Data integrity remains a cornerstone of FDA compliance, especially for medical devices where software increasingly drives product function. A data integrity program ensures that data is complete, consistent, and accurate throughout its lifecycle.
Core Components of Data Integrity Programs:
Policies and procedures aligned with FDA’s guidance
Training for personnel on data integrity principles
Regular data integrity assessments and audits
Implementation of technical controls like audit trails and access controls
Continuous monitoring and remediation of identified gaps
FDA Remediation: Addressing Compliance Gaps
FDA remediation refers to the corrective action’s companies undertake when non-compliance or deficiencies are identified through inspections, audits, or internal reviews. This process is essential to restore compliance and maintain market access.
Typical FDA Remediation Steps Include:
Identifying root causes of non-compliance
Implementing corrective and preventive actions (CAPA)
Updating procedures and training programs
Re-validating systems if necessary
Communicating transparently with the FDA when applicable
CSA FDA Guidance: Embracing the Future
The FDA’s draft guidance on CSA signals a shift in regulatory expectations, encouraging companies to adopt risk-based validation frameworks that leverage modern software development and testing methods.
By embracing CSA, medical device companies can:
Reduce time and cost of validation
Focus on high-risk software components
Use automation tools effectively
Improve overall software quality and patient safety
ALCOA+ Principles in Software Validation
ALCOA+ is an acronym representing the foundational principles of data integrity recognized by the FDA. These principles are critical in validating software used in medical devices and ensuring compliance.
Attributable: Data should clearly identify who recorded it and when.
Legible: Data must be readable and permanent.
Contemporaneous: Data should be recorded at the time the activity occurred.
Original: Data should be the original record or a certified true copy.
Accurate: Data must be free from errors.
The “+” adds additional principles like:
Complete: All data including metadata must be captured.
Consistent: Data should be logically consistent and conform to expected patterns.
Enduring: Data should be durable and retrievable over time.
Available: Data must be accessible for review and audit.
Incorporating ALCOA+ principles into software validation ensures that medical device data meets the highest standards of integrity and compliance.
Difference Between CSA and CSV: Summary Table
How Compliance Gurus Can Help
At Compliance Gurus, we specialize in helping medical device manufacturers navigate the complex FDA regulatory landscape. Whether you need assistance with CSV or CSA implementation, data integrity programs, or FDA remediation strategies, our expert consultants provide tailored solutions to ensure compliance and streamline your processes.
Our services include:
Comprehensive gap assessments
Development and implementation of data integrity programs
Risk-based software validation strategies aligned with CSA guidance
FDA remediation and CAPA management support
Training on ALCOA+ principles and compliance best practices
Frequently Asked Questions (FAQs)
Q1: What is the main difference between CSV and CSA?
A: CSV is a traditional, documentation-heavy approach to software validation, while CSA is a newer, risk-based methodology encouraged by the FDA to improve efficiency and focus on patient safety.
Q2: Why is data integrity important for medical device software?
A: Data integrity ensures that data is accurate, complete, and reliable, which is essential for patient safety, regulatory compliance, and product efficacy.
Q3: How does FDA remediation impact medical device companies?
A: FDA remediation involves correcting non-compliance issues identified by the FDA, which may include updating processes, retraining staff, and re-validating software, to maintain regulatory approval.
Q4: What are ALCOA+ principles?
A: ALCOA+ principles define data integrity criteria — Attributable, Legible, Contemporaneous, Original, Accurate — plus Complete, Consistent, Enduring, and Available, guiding compliance in data management.
Q5: Is CSA mandatory for FDA compliance?
A: CSA is currently recommended through FDA draft guidance as a best practice for risk-based software assurance, but CSV remains acceptable. CSA adoption is expected to grow.
Staying ahead in medical device compliance means adapting to new FDA expectations and regulatory approaches. By understanding the distinctions between CSV and CSA, implementing robust data integrity programs, and aligning with ALCOA+ principles, manufacturers can improve compliance, reduce risk, and enhance patient safety.
For expert guidance on all your medical device compliance needs, contact Compliance Gurus today.

Write a comment ...