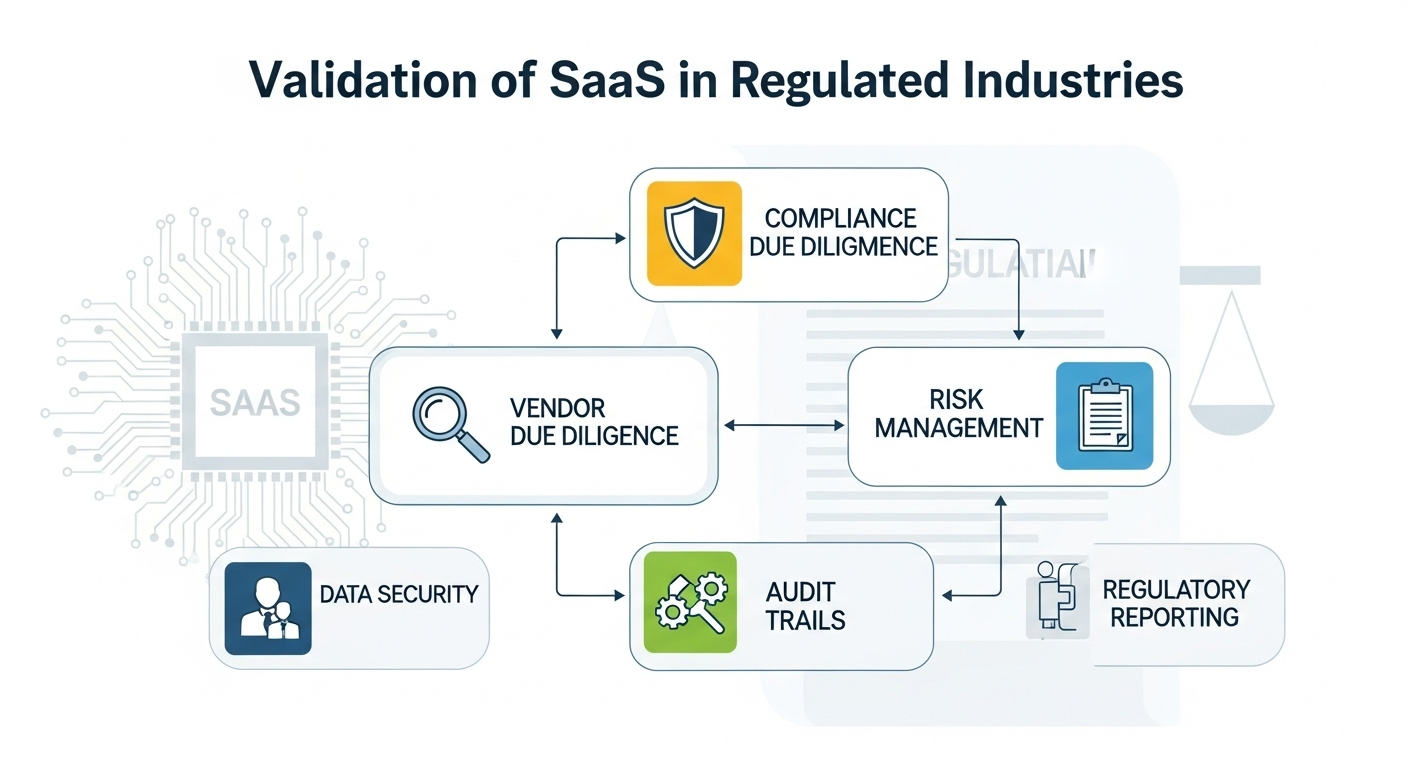
In today’s digital era, Software as a Service (SaaS) solutions are transforming how regulated industries operate. However, with innovation comes the responsibility of ensuring compliance, data integrity, and patient safety. Validation of SaaS applications in regulated Industries is not just a regulatory expectation—it’s a business necessity.
For industries like pharmaceuticals, medical devices, and biotech, validation ensures that SaaS platforms consistently perform as intended, maintain data accuracy, and comply with frameworks such as FDA 21 CFR Part 11, EU Annex 11, and GxP guidelines. Unlike traditional on-premise systems, SaaS solutions introduce unique challenges, including vendor dependencies, shared responsibilities, and frequent system updates.
A risk-based validation approach is critical to address these challenges. By focusing on critical processes and leveraging Computer Software Assurance (CSA) principles, organizations can streamline testing, reduce documentation burdens, and maintain continuous compliance.
At Compliance Gurus, we help companies navigate SaaS validation with proven strategies tailored to regulated environments. From vendor assessments to lifecycle documentation and change management, our approach ensures compliance while optimizing resources.
Validating SaaS is more than a checkbox—it safeguards trust, protects patients, and strengthens regulatory confidence. In regulated industries, compliance and innovation must go hand in hand.


Write a comment ...